Cincinnati, Ohio–May 9, 2022–Cincinnati-based Standard Bariatrics, Inc., an emerging leader in the bariatric surgery medical device field, closed a debt facility on April 27, 2022, with Silicon Valley Bank (SVB). The debt facility provides $7 million in term loans of which the first $4 million was funded at closing. The funding will be used to accelerate commercial efforts for the Titan SGS® surgical stapler and related proprietary medical devices designed for bariatric surgery.
Silicon Valley Bank is a leading investment bank partnering with innovative healthcare and technology companies. Under the terms of the new debt facility, SVB will make loans available in two tranches. The first tranche of $4 million was received at closing of which $0.4 million was used to retire existing debt. The second tranche of $3 million will be available through June 30, 2023, upon achieving certain milestones.
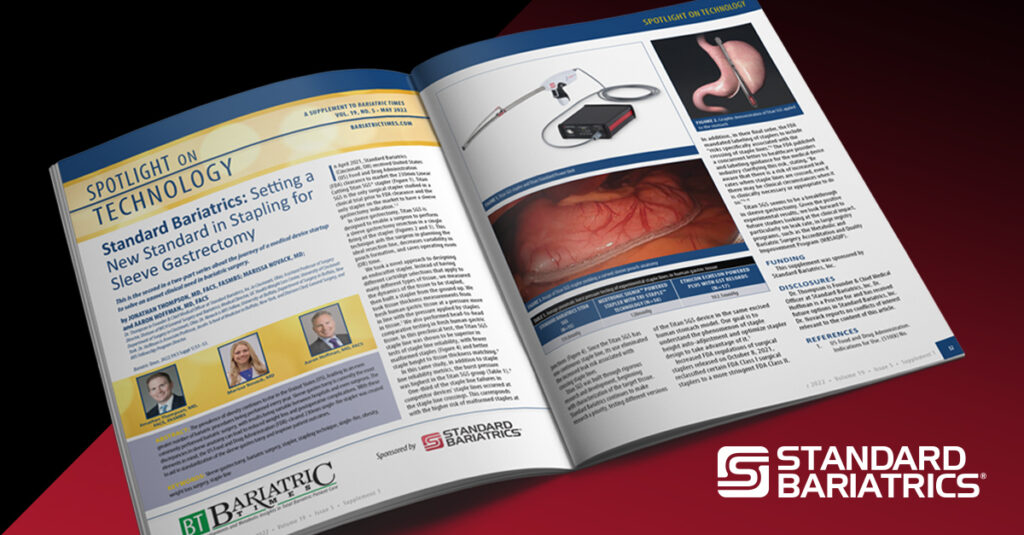
Cleared by the U.S. Food and Drug Administration (FDA) a year ago on April 28, 2021, the Titan SGS surgical stapler meets the new more stringent Class II product regulations for surgical staplers1. With a single firing of 55 seconds2 the Titan SGS stapler completes a 23cm staple line with no overlapping staples3. By completing the staple line in one continuous firing2, there is a reduced risk of anatomy variations associated with multiple, overlapping short-cartridge staple lines4.
Peter Donato, Chief Financial Officer at Standard Bariatrics, said, “We are pleased to continue our long-standing relationship with Silicon Valley Bank as we continue to exceed our sales and production expectations. While our balance sheet is very strong with the recent Series B equity raise, we are adding this debt facility to allow for greater speed and flexibility to accelerate our commercial efforts, further securing our supply chain and scaling our administrative capabilities.”
Karen Spilizewski, Vice President with RiverVest® and a Board Member at Standard Bariatrics said, “The clinical and technical advantages of Titan SGS are resonating with bariatric surgeons and the community of users continues to expand quickly. Early results from the 2,500 cases completed to date give us the confidence to keep investing in Titan SGS and its adjunct technologies. This debt facility will accelerate the organization’s progress toward new development milestones.”
About Standard Bariatrics, Inc.
Standard Bariatrics, Inc., is a rapidly expanding medical device company focused on the development and commercialization of products for the surgical treatment of obesity. Driven by a passionate group of surgical innovators, the Cincinnati-based organization works hand in hand with bariatric surgeons to develop and release novel solutions designed to address the growing global epidemic of obesity. The Standard Bariatrics team offers extensive experience in creating and bringing to market bariatric-related medical device technologies. They have a demonstrated record of achieving clinical excellence with economically responsible solutions for providers and their patients.
Standard Bariatrics is supported through early investment from Jobs Ohio, North Coast Angel Fund, Accelerant, Queen City Angels, CincyTech, Series A lead investors RiverVest®, Hatteras Venture Partners and Emergent Medical Partners, and Series B investors U.S. Venture Partners (USVP) and RC Capital.
About Silicon Valley Bank
For nearly 40 years, Silicon Valley Bank (SVB) has helped innovative companies and their investors move bold ideas forward, fast. SVB provides targeted financial services and expertise through its offices in innovation centers around the world. With commercial, international and private banking services, SVB helps address the unique needs of innovators. Learn more at svb.com.
- FDA. (2021, October 7). FDA issues final order and guidance on Surgical Staplers and staples for internal use. U.S. Food and Drug Administration. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-issues-final-order-and-guidance-surgical-staplers-and-staples-internal-use
- U.S. Food and Drug Administration. (2021). Indications for Use. (510(k) No. K210278). Retrieved from https://www.accessdata.fda.gov/cdrh_docs/pdf21/K210278.pdf
- Salyer, C., Spuzzillo, A., Wakefield, D., Gomaa, D., Thompson, J., & Goodman, M. (2020 July). Assessment of a novel stapler performance for laparoscopic sleeve gastrectomy. Surgical Endoscopy, 35(7), 4016–4021. https://doi.org/10.1007/s00464-020-07858-0
- Toro, J., Lin, E., Patel, A., Davis, S., Sanni, A., Urrego, H., Sweeney, J., Srinivasan, J., Small, W., Mittal, P., Sekhar, A., & Moreno, C. (2014 Sept.). Association of Radiographic Morphology with Early Gastroesophageal Reflux Disease and Satiety Control after Sleeve Gastrectomy. Journal of the American College of Surgeons, 219(3), 430–438. https://doi.org/10.1016/j.jamcollsurg.2014.02.036
Media Contact
Ronald Galovich
Chief Commercial Officer, Standard Bariatrics
513.620.7751
ron@standardbariatrics.com