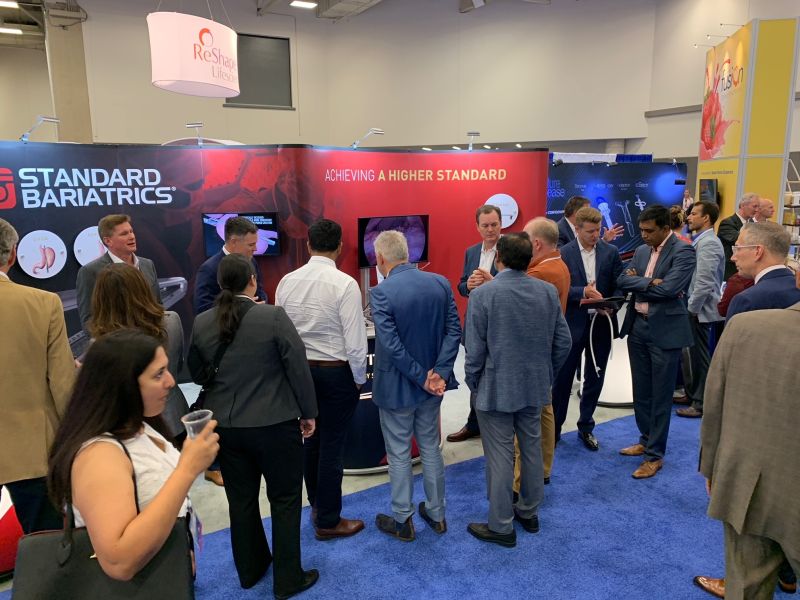
Got a minute? That’s all it takes with Titan SGS®. Learn more in ASMBS Booth #509!
Stop by Booth #509 here at ASMBS in Dallas and learn more about the only stapler indicated to complete sleeve pouch anatomy in one 55-second firing cycle1.
Surgeon users are at the booth and eager to talk about how Titan SGS is helping them achieve repeatable sleeve pouch anatomy. Learn first-hand how Titan SGS technology may save time in the OR and reduce the cost of care.
Also, visit Business Suite #1 and check out our showcase of future innovations for bariatric surgery.
See you soon!
Learn more about Standard Bariatrics and request training on Titan SGS at standardbariatrics.com.
1. U.S. Food and Drug Administration. (2021). Indications for Use. (510(k) No. K210278). Retrieved from https://lnkd.in/ggJ7N4Yf
#thestandardsleeve#sleevegastrectomy#bariatricsurgery#standardbariatrics#thestandardsleeve