Shape matters.
Consistency matters.
Outcomes matter.
Discover the value the Titan SGS™ Stapler can deliver for you.
Just
Published
New retrospective study in Obesity Surgery reports perioperative outcomes of the Titan SGS™ Stapler
Propensity matched retrospective review of 783 laparoscopic sleeve gastrectomies reports the perioperative outcomes of the Titan SGS™ Stapler as compared to multi-fire surgical staplers. The study results link the Titan SGS™ Stapler to potentially enhanced outcomes. The study was conducted by surgeons at Corewell Health Hospital in Grand Rapids, MI.
Dr. Schram and Dr. Foote, two of the authors of the study, are paid consultants of Teleflex Incorporated. Fritz, G.D., Sharrak, A., Aubrey, J. et al. Perioperative Outcomes Using Single-Fire Stapler. OBES SURG (2024). https://doi.org/10.1007/s11695-024-07357-4




Retrospective study shows potential improvement in GERD and nausea.4,8
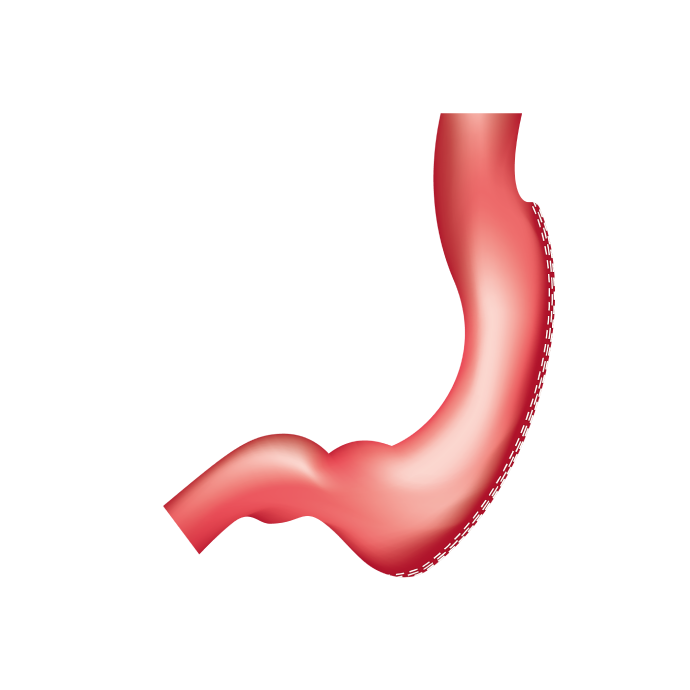
Standard Sleeve™ Technique
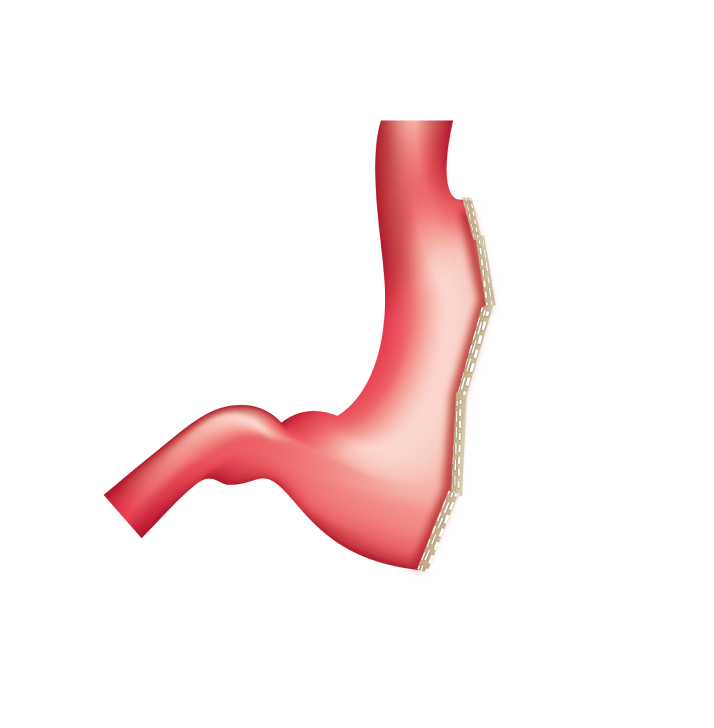
Multiple-fire technique
Economics & Efficiencies
The Titan SGS™ Stapler takes an anatomy-based approach to sleeve gastrectomy using the Standard Sleeve™ Technique for a more consistent, symmetrical sleeve pouch anatomy.1 The 23cm, single-fire stapler is designed to help you standardize operational efficiency and patient outcomes.
The Titan SGS™ Stapler offers unique strengths to align with the IHI Triple Aim Initiative and support your goals in cost savings, quality of care, and clinical outcomes. Take a look at the potential benefits for surgeons, hospital systems and patients, based on recent studies.


27% reduction in OR time, from 63 to 46 minutes.*4
*Compared to other reviewed commercially available staplers and equipment sets.


Check back often for the latest outcomes associated with the Titan SGS™ Stapler as you and your VAC make decisions about the most effective tools for sleeve gastrectomy.
Want to stay informed of the latest developments?
Learn more about the value of the Titan SGS™ Stapler for you, your patients and your organization!
Contact your sales representative.
Enhance your Standard Sleeve™ Technique
Become a credentialed user of the Titan SGS™ Stapler today.
No cost and no obligation!
1. 510(k) No. K210278. The Titan SGS linear cutter is intended for longitudinal transection and resection of gastric tissue for sleeve gastrectomy pouch creation. 2021.
2. Varban, O. A., Niemann, A., Stricklen, A., Ross, R., Ghaferi, A. A., Finks, J. F., & Dimick, J. B. (2017, Aug.). Far from Standardized: Using Surgical Videos to Identify Variation in Technique for Laparoscopic Sleeve Gastrectomy. Journal of Laparoendoscopic & Advanced Surgical Techniques. Volume 27, Number 8.
3. Salyer, C. E., Thompson, J., Hoffman, A., Burstein, M. D., Enochs, P., Watkins, B. M., Kuethe, J., & Goodman, M. D. (2022). Multisite Study of Titan SGS Stapler in longitudinal gastric resection. Surgical Endoscopy. https://doi.org/10.1007/s00464-022-09051-x
4. Standard Bariatrics, Inc. Multisite comparison of Titan SGS™ Stapler to existing surgical staplers in sleeve gastrectomy. (Unpublished raw data). Qualitee 360 Report. 2022. Retrieved from Metabolic and Bariatric Surgery Accreditation and Quality Program database.
5. 1999–2021 American Hospital Association Annual Survey. Copyright 2021 by Health Forum, LLC, an affiliate of the American Hospital Association. Accessed 18 May 2023. Expenses and cost savings will vary by facility.
6. Standard Bariatrics, Inc. (October 2023) Multisite comparison of Titan SGS™ Stapler to existing surgical staplers in sleeve gastrectomy. (Unpublished raw data). Qualitee 360 Report. Retrieved from Metabolic and Bariatric Surgery Accreditation and Quality Program database.
7. Healthcare Cost and Utilization Project. (2021). Overview of Clinical Conditions With Frequent and Costly Hospital Readmissions by Payer, 2018. Statistical brief #278.Agency for Healthcare Research and Quality, Rockville, MD. https://hcup-us.ahrq.gov/reports/statbriefs/sb278-Conditions-Frequent-Readmissions-By-Payer-2018.jsp Accessed 3 October 2023. Expenses and cost savings will vary by facility.
8. Thompson, J., Dhar, V., Hanseman, D., Watkins, B., Morton, J., & Diwan, T. (2017). Anatomy-based laparoscopic sleeve gastrectomy reduces gastroesophageal reflux disease compared to laparoscopic sleeve gastrectomy with bougie. Surgery for Obesity and Related Diseases, 13(10). https://doi.org/10.1016/j.soard.2017.09.242
9. U.S. Food and Drug Administration Center. (2021, Oct. 8). Surgical Staplers and Staples for Internal Use – Labeling Recommendations. (Docket No. FDA-2019-D-1262). Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/surgical-staplers-and-staples-internal-use-labeling-recommendations
10. Data on file at Teleflex Incorporated.
11. Jung, J. J., Jackson, T., Gordon, L., & Hutter, M. M. (2022). Intraoperative leak test is associated with lower postoperative bleed rate in primary sleeve gastrectomy: A propensity matched analysis of primary and revision bariatric surgery using the MBSAQIP database. Surgical Endoscopy, 36, 753–763. https://pubmed.ncbi.nlm.nih.gov/33475846/
12. Purich, K., Mocanu, V., Joy, J., Verhoeff, K., Dang, J., Switzer, N. J., Birch, D. W., & Karmali, S. (2022). The impact of metabolic and bariatric surgeon status on outcomes after bariatric surgery: A retrospective cohort study using the MBSAQIP database. Obesity Surgery, 32(6), 1944–1953. https://pubmed.ncbi.nlm.nih.gov/35349044/
Rx only.
Teleflex, the Teleflex logo, Standard Bariatrics, Standard Sleeve, and Titan SGS are trademarks or registered trademarks of Teleflex Incorporated or its affiliates in the U.S. and/or other countries. Other names are trademarks of their respective owners. Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries. Please contact your local representative. Before using any medical device review all relevant package inserts with particular attention to the indications, contraindications, warnings and precautions, and steps for use of the device.
- © 2026 Teleflex Incorporated. All Rights Reserved. MC-010007 Rev 1 10/24
