NEWS
Credibility and confidence
November 8, 2024
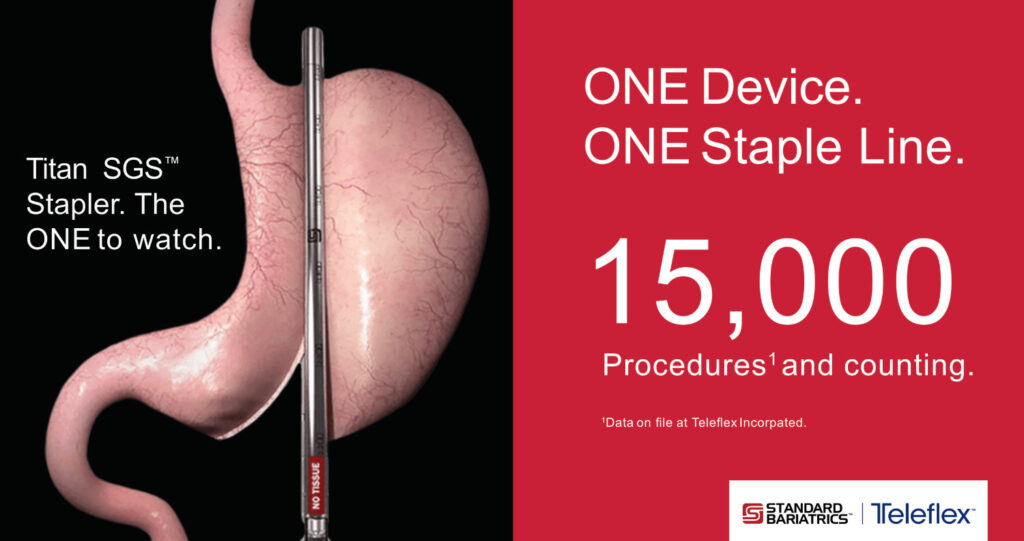
Surgeons have completed more than 15,000 sleeve gastrectomy procedures using the Titan SGS™ Stapler.1
1.Data on file at Teleflex Incorporated.

New study reports perioperative outcomes of the Titan SGS™ Stapler
November 1, 2024
Here’s exciting news for patients and surgeons: a study published in Obesity Surgery in August 2024 links our Titan SGS™ Stapler to potentially enhanced perioperative outcomes compared to multi-fire surgical staplers.

Welcoming Andrew M. DeWitt, MD, to the Titan SGS™ Community of Users
We are proud to welcome Andrew M. DeWitt, MD, FACS, FASMBS, FPD-MBS, and his team in Alabama to the community of Titan SGS™ users. After using Titan SGS, Dr. DeWitt said the staple line was as “clean as a whistle.” Dr. DeWitt has been practicing in bariatric surgery for over

We enjoyed seeing you in Italy!
Grazie! We appreciate the time you spent with us at IFSO 2023. We hope you enjoyed learning about Titan SGS™, a 23cm, single-fire stapler for the Standard Sleeve™ technique, an anatomy-based approach to sleeve gastrectomy that enables a more consistent and symmetrical sleeve anatomy. To learn more about Titan SGS™

A Warm Thank You to the Mitten State!
Thank you to the Michigan Bariatric Society! It was an incredible day learning from the who’s who of bariatric surgery that gathered from across the country for the annual chapter meeting. Your dedication to improving patient outcomes by pursuing quality care and clinical research helps improve the management of obesity.

See you in Italy!
Teleflex™ is excited to attend the International Federation for the Surgery of Obesity and Metabolic Disorders World Congress in Napoli, Italy, from August 30 to September 1. We look forward to engaging with the global community of bariatric surgeons. Please stop by the Teleflex booth to learn about Titan SGS™,

We loved visiting Virginia!
Thank you to the Virginia Bariatric Society. We enjoyed exhibiting and showcasing the Titan SGS™ stapler last week in Williamsburg, VA. Titan SGS™ is a 23cm, single-fire stapler for the Standard Sleeve™ technique, an anatomy-based approach to sleeve gastrectomy that enables a more consistent and symmetrical sleeve anatomy. Additional thanks to special

Georgia, you’re a peach!
It was a pleasure to meet the bariatric surgeons at the 2023 annual meeting of the Georgia Society of the American College of Surgeons and Obesity Day hosted last week on the beautiful St. Simons Island. Thank you for your interest in learning about the Standard Sleeve™, an anatomy-based approach
